What is Zeta Potential?

Zeta potential is a measure of charges carried by particles suspended in a liquid (mostly water).
Most particles suspended in water and wastewater, e.g. clays, silica, hydrated metal oxides, paper fibers, biological cells, etc., possess negative surface charges in the neutral pH range as shown in Fig. 1 (a simplified view of a particle in a suspension). The particle is said to carry a negative electrostatic surface charge. Positive ions in the solution are attracted to the negatively charged surface where they may be strongly adsorbed. The adsorbed layer remains rigidly attached and forms what is known as the Stern layer. Outside the Stern layer is a diffuse layer in which positive ions outnumber negative ions and balance the excess in the Stern layer. The electrostatic potential at the particle surface A decreases through the Stern layer along AB and along BC in the diffuse layer and reaches zero at point C where the bulk of the solution is encountered. The Stern layer and diffuse layer are called double layers. They are important in determining forces between colloidal particles. The Zeta Potential BC is the difference between the charge at the edge of the Stern layer and the bulk of the suspending liquid as shown in Fig 1.
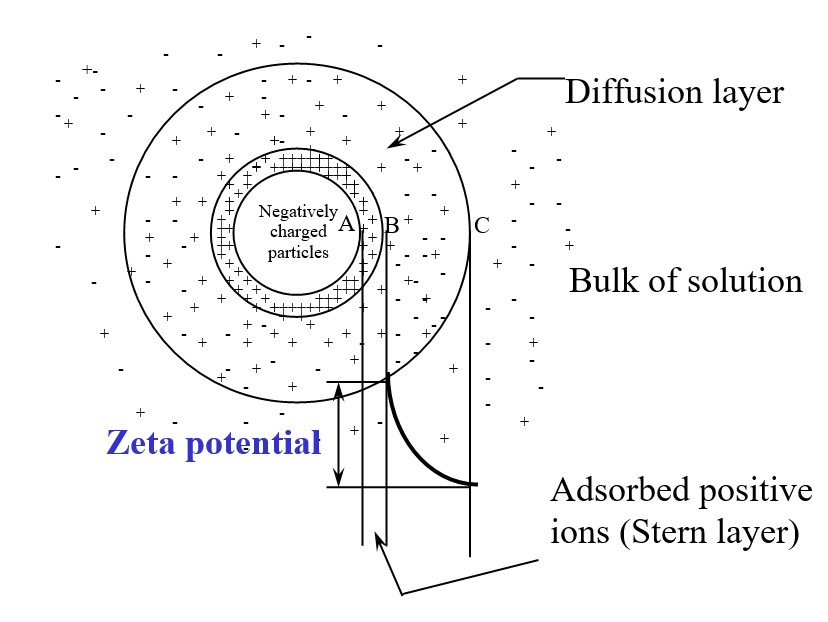
Table 1: Stability of suspensions with relation to Zeta Potential (Riddick, 1968)
Stability Characteristics | Avg. Zeta Potential in mV |
Maximum agglomeration and precipitation | 0 to +3 |
Range of strong agglomeration and precipitation | +5 to –5 |
Threshold of agglomeration | -10 to –15 |
Threshold of delicate dispersion | -16 to –30 |
Moderate stability | -31 to –40 |
Fairly good stability | -41 to –60 |
Very good stability | -61 to -80 |
Extremely good stability | -81 to -100 |
Zeta Potential can be measured by tracking the moving rate of a negatively or positively charged particle across an electric field. The commercial instrument is a zeta meter which is quite costly and requires some experiences to get reliable measurement.
Small particles do not form agglomerates when there are large mutually repulsive electrostatic charges. Coagulation generally refers to agglomeration by charge neutralization and can be controlled by zeta potential in relation to the stability of suspensions as shown in Table 1.
Zeta Potential values less negative than –15 mV usually represent the onset of agglomeration. The threshold region of either coagulation or dispersion exists from about –14 mV to –30 mV. Values more electronegative than –30 mV generally represent sufficient mutual repulsion to result in stability (i.e. no agglomeration).
For more information, one can attend one of the short courses offered by the American Filtration and Separation Society.