Hydrocarbon Evaporative Emissions & Control for Automotives, Part 1 – Adsorption & Activated Carbon

Even the cleanest and most efficient vehicle on the market today still pollutes the air and otherwise damages the environment. Motor vehicles emit several noxious pollutants which vehicle emissions standards are designed to regulate. Vehicles generally emit Hydrocarbons (HC), Carbon monoxide (CO), Nitrogen oxides (NOx) and Fine airborne particulate matter (PM) as pollutants that require control. California has been at the forefront of environmental rulemaking affecting the automotive industry. The California Air Resources Board (CARB) is the regulatory body that administers the state regulations, as does the United States Environmental Protection Agency (EPA) at the federal level. Part 1 of the series deals with understanding adsorption and using activated carbon to remove hydrocarbons.
Adsorption
Adsorption, the binding of molecules or particles to a surface (must be distinguished from absorption), the filling of pores in a solid. The binding to the surface is usually weak and reversible. Just about anything including the fluid that dissolves or suspends the material of interest is bound, but compounds with color and those that have taste or odor tend to bind strongly. Compounds that contain chromogenic groups (atomic arrangements that vibrate at frequencies in the visible spectrum) very often are strongly adsorbed. The most common industrial adsorbents are activated carbon, silica gel, and alumina, because they present enormous surface areas per unit weight. Activated carbon is produced by roasting organic material to decompose it to granules of carbon – coconut shell, wood, and bone are common sources. Silica gel is a matrix of hydrated silicon dioxide. Alumina is mined or precipitated aluminum oxide and hydroxide.
Activated Carbon
Activated carbon is by far one of the most popular mass produced adrosption materials. Acticvated carbon is typically, powdered, granular, or pelleted form of amorphous carbon characterized by very large surface area per unit volume. The large surface area is due to the enormous number of fine pores. Activated carbon is capable of collecting gases (fuel vapors, VOCs), liquids, or dissolved substances on the surface of its pores.
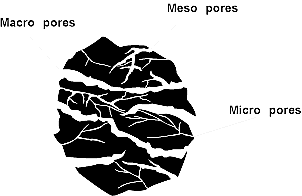
Adsorption on activated carbon is selective, favoring nonpolar over polar substances. Compared with other commercial adsorbents, activated carbon has a broad spectrum of adsorptive activity, excellent physical and chemical stability, and ease of production from readily available materials. The adsorption process takes place in three steps: (see figure 1)
- Macro pore transport: The movement of organic material through the macro-pore system of the active carbon (macro-pore >50nm). The large macro-pores act as channels for molecules to transfer through the carbon to the meso and micro pores
- Meso pore transport: (medium pores) with diameters of 2-50 nm where adsorption takes place
- Micro pore transport: The movement of organic material through the meso-pore and micro-pore system of the active carbon (micro-pore <2nm). The micro pores where adsorption largely takes place.
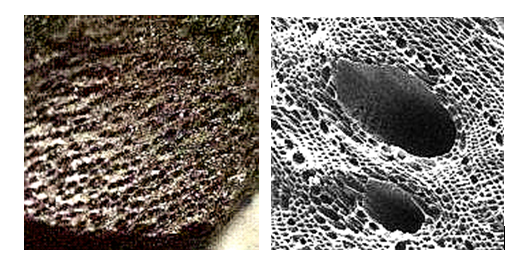
- Sorption: The physical attachment of organic material on the surface of active carbon in the meso-pores and micro-pores of the active carbon. Figure 2 shows actual SEM pictures of the porous structure of activated carbon.
Almost any carbonaceous raw material can be used for the manufacture of activated carbon. Wood, peat, and lignite are commonly used for the decolorizing materials. Bone char made by calcining bones is used in large quantity for sugar refining. Nut shells (particularly coconut), coal, petroleum coke, and other residues in granular, briquetted, or pelleted form are used for adsorbent products.
Activation
Activation is the process of treating the carbon to open an enormous number of pores in the 1.2- to 20-nanometer-diameter range (gas-adsorbent carbon) or up to 100-nm-diameter range (decolorizing carbons). After activation, the carbon has the large surface area responsible for the adsorption phenomena. Carbons that have not been subjected previously to high temperatures are easiest to activate. Selective oxidation of the base carbon with steam, carbon dioxide, flue gas, or air is one method of developing the pore structure. Other methods require the mixing of chemicals, such as metal chlorides (particularly zinc chloride) or sulfides or phosphates, potassium sulfide, potassium thiocyanate, or phosphoric acid. A gram of activated carbon may have a surface area in excess of 400 m², with 1500 m² being readily achievable. For comparison, a tennis court is about 260 m²
References
- Development of Evaporative Emissions Filters for Automotive Applications, J.M. Leffel and G.S. Green, American Filtration and Separations Society Conference, Reno, Nevada, June 17-20, 2003.
- Jeffry M. Leffel and Reza Abdolhosseini, PhD, ‘Requirements Setting, Optimization and “Best Fit” Application of AIS Hydrocarbon Adsorption Devices for Engine Evaporative Emissions Breathing Loss Control”, SAE technical paper #2005-01-1104, presented in Detroit 2005.
- Schaffer S, Arruda A., Bielicki J. And Bugli N., ‘Design Considerations and Characterization Test Method for Activated Carbon Foam Hydrocarbon Traps in Automotive air Induction Systems’, Paper presented at the 2007 SAE Conference and Expo, #07PFL-913, Detroit, MI.
- http://www.lenntech.com/adsorption.htm
- http://www.answers.com/topic/activated-carbon