Amine Unit Contamination Overview

Amine Units play a key role in today’s refinery operations and gas plants in this quest for lower sulphur emissions and improved safety. It is thought that a well operated Amine Unit has no need for amine filtration. This might be the case in an ideal system; however, these are seldom encountered. Therefore, filtration and a few other separation technologies (see future Part 2) are not only the last line of defense for an Amine Unit or any other plant for that matter, but might also be the only line of protection. It is then to any plant’s advantage to deploy the best and more advanced contamination control devices to ensure process stability, equipment reliability and enhanced throughput. Proper separation technologies can mitigate some of the most common problems found in the Amine Units (and TGT Units) such as:
Fouling
This is deposition of solids and hydrocarbons along with other components to form a coating over equipment surfaces, typically in hot, low velocity locations such as heat exchangers and reactor columns. Fouling has many formation mechanisms, but there is some agreement that is has two components, 1) a free radical polymerization or decomposition of dissolved species present in the stream, or 2) deposition of suspended matter in the stream. Today, fouling is generally prevented by chemical means (e.g. free radical inhibitors such as hindered phenols) or by mechanical means, such as filtration. Fouling leads to energy losses and flow restrictions and is a source of major maintenance expense.
Corrosion
Corrosion is part of the daily life in Amine Units, and correct filtration is crucial to minimizing suspended solids. Typically where there is fouling, the next natural progression is corrosion under any deposited solids caused by local, elevated concentrations of corrosion initiators under that material. Usually if fouling is minimized, corrosion rates will be lowered as well. A similar phenomenon, erosion corrosion, also occurs from the impact of hard and dense suspended particles, such as carbon residues, with equipment surfaces.
Heat Stable Salts
Heat-stable salts are components in the amine solutions, which hinder reversal of the formation reaction and do not decompose under normal regenerative conditions. Some salts are not entirely “heat stable”, but instead stable under the available process conditions. It is believed that many of these salts are formed at an accelerated rate due to the extended surface provided by suspended solid contaminants and mediated by metal ions in solution such as iron. Solid surfaces possess considerable active sites for heterogeneous reactions and are also rich in metals species. These can catalyze salt formation reactions, rapidly increasing the concentration of heat stable salts as well as promoting amine decomposition.
Foaming
Foam is generally produced by the association of gases and liquids stabilized by surfactants that lower surface tension. The surfactants are surface-active substances, acting at the interface of oil/water boundaries. They can be solid particles (e.g. micron and sub-micron iron sulphides) or molecules (e.g. compressor lubrication oils – lubrication oils in general have many are surface-active molecules in their formulation). Removal of solids, hydrocarbons and other surface-active agents greatly reduce foaming events and the use of antifoam additives. Foaming invariably leads to amine loss and lower sweetening efficiency. Other factors that lead to amine foaming have several, common root-causes. Therefore, a proper evaluation by the plant of its process, equipment and feed composition is required to determine the source and best means of mitigation.
Regenerator/Absorber Protection and Amine Solution Quality
Filtration and separation systems on the amine-rich stream are designed to protect the heat exchanger and regeneration section of an Amine Unit and to protect the Sulphur Recovery Unit (SRU) by facilitating the delivery of good quality acid gas. This is done by ensuring that the amine is free of contaminants that might foul the rich/lean heat exchanger causing added duty for the reboiler and leading to possible corrosion episodes. More plants are implementing filtration of the amine-rich stream as well as the amine-lean stream for iron control. In fact, both filtrations are not only necessary, but are complementary to each other since they function in different modes. The fundamental functions of lean amine filtration is to remove suspended solids and to separate dissolved components via the activated carbon bed, thereby ensuring that a clean amine solution is delivered to the absorber.
Low Efficiency
Filtration is designed to remove suspended solids from the amine solution. It is known that an amine solvent with high solid contents produces less efficient H2S/CO2 phase transfer and absorption. This is caused by the multilayer arrangement of solids at the interface of the gas (or liquid) and the amine solution, essentially hindering mass transfer. Good quality amine solution with minimal contaminants yields a more stable process and prevents equipment damage.
Conclusions
Perhaps the most relevant learning over years of field experience is that a key step in process control is proper contamination control. Most plants that do not take this critical step, struggle with high operational costs and low systems reliability, in addition to numerous detrimental technical, economic and environmental effects. There is no significant disadvantage to implementing enhanced separation and filtration apart from a marginal increase in capital costs. One might tend to believe that total cost will be prohibitive, but experience shows that this is not the case and operational costs can actually be lower. 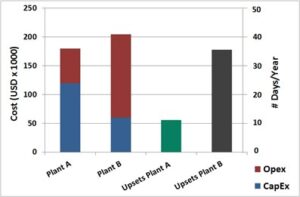 On the other hand, there are real and serious issues involved with neglecting separation and filtration systems or using systems with deficient designs, low cost systems and not giving the proper attention to contamination control. Invariably, any capital savings in low cost separation and filtration will ultimately lead to exponentially high processing costs, low reliability and frequent unit upsets. The included figure illustrates such situation. The chart above compares two actual cases and historical operating and capital data from two fairly similar Amine Units. As one can seee, low capital expenditures (CapEx) do not necessarily translate into lower overall cost due to much higher operational cost (OpEx). Additional consequences are the significant negative economic impact of much more frequent plant upsets (measured here in days the plant was in upset mode each year).
On the other hand, there are real and serious issues involved with neglecting separation and filtration systems or using systems with deficient designs, low cost systems and not giving the proper attention to contamination control. Invariably, any capital savings in low cost separation and filtration will ultimately lead to exponentially high processing costs, low reliability and frequent unit upsets. The included figure illustrates such situation. The chart above compares two actual cases and historical operating and capital data from two fairly similar Amine Units. As one can seee, low capital expenditures (CapEx) do not necessarily translate into lower overall cost due to much higher operational cost (OpEx). Additional consequences are the significant negative economic impact of much more frequent plant upsets (measured here in days the plant was in upset mode each year).