Coalescing Process

Coalescing is accomplished using a device for the separation of a liquid/liquid emulsion of dispersed small discrete droplets from a basically unstable fluid where gravity alone is not sufficient to make the separation.
The coalescing process separates emulsions of two intimately dispersed, immiscible liquids, by passing them through a deep-bed fiber matrix such as a nonwoven fabric, felt or fiber mat. In an emulsion, such as water (discontinuous phase) contaminated fuel (continuous or primary phase) passes through the medium, water drops are intercepted when they impact fibers of the medium. Surface tension causes the water droplets to be stripped from the emulsion and to be captured by the fibers and to each other. As more water droplets impact those already captured, they continue to grow together or coalesce until the droplets reach such a mass that they become dislodged from the fibers and eventually, as the heavier of the two phases, drop with gravity. The lighter fuel phase migrates through the coalescing device and ultimately rises to complete the separation from the water phase. Conversely, should water be the continuous phase, then the fuel would be coalesced from the water.
As a rule, coalescers begin to lose efficiency when the difference of any two constituent fluids interfacial tensions (IFT) fall below 18-20 dynes/CM and become very difficult under 6-8 dynes/CM. The key is to use an optimum fibrous medium when separating low and/or close IFT fluids. Efficient coalescence also requires the coalescing medium be chemically compatible with the fluids being coalesced.
Coalescers are sometimes designed as a multi-stage system. The first stage, is typically a pleated element, which serves to act as a prefilter to remove solid particulate, which otherwise might plug the coalescers pores. For difficult to extremely pure separations, distillation process are sometimes employed following a coalescer to complete a process.
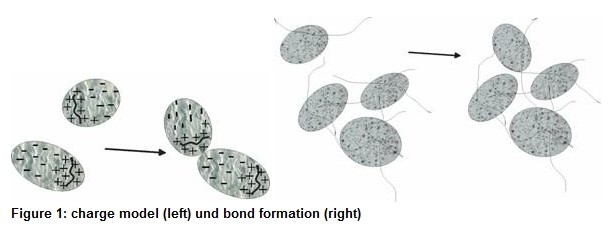
To reach an actual agglomeration the destabilized particles need to collide with each other or get in range of mutual attraction.
During the transport process, depending on whether diffusive transport of small particles (x<1m) or inertial transport of large particles (x>1m) predominates, one talks of perikinetic or orthokinetic transport. Responsible for the movement during perikinetic transport is the Brownian motion. In the case of orthokinetic transport the larger particles cannot follow the liquid flow due to their inertia and collide with each other.
Macroscopically destabilization and transport are happening simultaneous. The result of a flocculation experiment is difficult to predict. The mechanisms depend on the material system and treatment of the sample. They are highly variable and have many influencing parameters such as amount and duration of energy input, concentration and addition mode of flocculant, stirrer, pH, etc..
The life span and size of a flake is primarily determined by the entry of shear forces and centrifugal forces and the nature of the formation of the flake. The strength is influenced by the formation mechanism. The lowest binding forces have flakes, which were generated by changing the physico-chemistry, the highest strength flakes result from the addition of polymeric flocculants.